Lymphoma treatment can vary significantly, depending on the type of lymphoma and the patient. The treatment for non-hodgkin lymphomas differs from one type of lymphoma to the next. The treatment may be different for an elderly patient or a young person with an aggressive form of lymphoma. It also depends on what stage the lymphoma is at.
The main types of treatment for lymphoma comprise:
Chemotherapy
Using drugs to kill the cancerous cells. Chemotherapy (‘chemo’) inhibits cell division and thereby prevents the cancerous cells from proliferating. Regimens such as R-CHOP, R-ICE, R-DHAP and R-GemOx are widely used as first-line treatments.
Immunotherapy
Using the patient’s own immune system to fight the cancer. Monoclonal antibodies are an example of effective immunotherapy treatments for certain types of lymphoma.
Radiotherapy
Using radiation to destroy the cancerous cells as they divide, as is done in the treatment of lymph node cancer.
Cell therapy
Replacing cancerous cells with healthy ones, as in the case of autologous stem cell transplantation.
Targeted therapies
Using drugs to target specific anomalies in cancerous cells, helping improve the lymphoma treatment.
For more information about a specific treatment, speak to your doctor or consult the dedicated sections on this website.
Non-Hodgkin lymphoma is very often treated nowadays in the first line using immunochemotherapy, i.e. a combination of chemotherapy and a biological treatment.
Chemotherapy inhibits cell division, including the division of cancerous cells. You may recognise the names R-CHOP, R-ICE, R-DHAP, R-GemOx and others. Don’t hesitate to speak to your doctor for more details.
Immunotherapy makes it possible to target the disease cells for example by using an antibody.
Process
Chemotherapy is generally administered in the hospital as an outpatient treatment.
Side effects

The side effects and possible complications of immunochemotherapy include:
The side effects and possible complications of immunochemotherapy include:
For more information about a specific treatment, speak to your doctor or consult the dedicated sections on this website.
Radiotherapy, which is often used after chemotherapy, targets the cancerous cells that are dividing, compromising their ability to proliferate. It does so using ionising radiation. Although it can affect the healthy tissues, they are able to recover between the sessions. The overall radiation dose is administered in several smaller doses over a variable period. Each session lasts about ten minutes and the treatment is not painful; it does not make you radioactive.
Side effects
The usual side effects of radiotherapy:
depend on the area of the body being treated.

Advice about diet, personal care and administration of medication can help effectively in avoiding and/or ameliorating these side effects.
Immunotherapy is the term covering all treatments that utilise the immune system for treating illnesses. Immunotherapy for cancer includes various strategies and techniques that manipulate the immune system to yield a more effective immune response against malignant cells.

Download the immunotherapy brochure for more information about the immune system.
These treatments include:
Each of these methods aims to boost the organism’s immune response to cancer, allowing it to be controlled better or even cured.
These are drugs that can reactivate the immune system in cases where it has been deactivated by the tumour.
How it works
The cancerous cells may be giving off certain inhibitory signals to the T cells that prevent them from being activated. Inhibiting the T cells lets the cancerous cells prevent the immune system from attacking them. Checkpoint inhibitors can disable that inhibitory effect. In some patients, this in turn lets the immune system start attacking the tumour again.
For more information download the immunotherapy brochure.
Bispecific antibodies bind to two targets, boosting the immune response against the cancer. Binding to two or more targets lets them identify and destroy cancerous cells. Generally, there is at least one target on a cancerous cell and one type of immune system cell that is stimulated to kill the cancer cells. One typical example is BiTEs, which binds to T cells. Trispecific antibodies are able to bind to an additional antigen on the organism’s T cells, or to two cancerous cells or two T cells; this leads to additional activation and more effective elimination of the cancer cell.
For more information download the immunotherapy brochure.
Monoclonal antibodies specifically target and destroy cancerous cells. They can bind to the antigens that are present on the cancer cell surface and trigger an immune response that will destroy those cells.
How it works
They are synthetic antibodies that bind to a specific target or antigen in order to destroy the cancerous cell. Directly attaching a synthetic antibody to the cancer cell acts as an alarm signal that alerts the immune system and thereby destroys the cancer cell. Another way of activating the immune system using antibodies consists of suppressing the inhibitory checkpoints that were used by the cancer cells in order to suppress the immune system (this technique is discussed separately, though). Moreover, antibodies can also occupy and block growth receptor sites on the surface of the cancer cell and thereby block cell division. This can also be done indirectly using antibodies that target the growth signals that are present in the cancer cell’s surroundings.
Antibodies linked to a toxic substance or to radiation. A final and ingenious way of destroying cancer cells using synthetic antibodies involves linking the antibody to a toxic substance such as a chemotherapy agent, a cytokine or a form of radiation, thereby ensuring that it is administered in a targeted way to the cancer cell.
To learn more about immunotherapy, download our brochure.
Administering stem cells from a donor (allogeneic) which transform into a new immune system.
How it works
In the case of an allogeneic stem cell transplant, the patient’s immune system and bone marrow are first largely destroyed by a combination of chemotherapy and/or radiotherapy and/or antibodies. This intense pre-treatment may also destroy certain residual cancerous cells, but the main purpose is to render the patient’s bone marrow and immune system receptive to stem cells from the donor and to prevent the donated cells (which are foreign to the body) from being rejected. Furthermore, to avoid the donor cells being rejected, it is also important to use stem cells from a donor with the same tissue type. Genetic heredity plays a role in this, so the first step is to look for a suitable donor (HLA-identical) from within the immediate family (siblings). If one cannot be found, a worldwide donor bank can be consulted. If this search is also fruitless or is taking too long, it is also possible to look for semi-identical donors (haplo-identical) within the family. Finally, umbilical cord blood is also an option.
The donor’s healthy stem cells become a new immune system that recognises and attacks the cancer cells. Large numbers of mature immune cells are administered at the same time as the stem cells.
For some aggressive conditions, this is the only option for curing the disease. However, it is an extremely high-burden treatment with a high risk of serious or even fatal complications.
For more information download the immunotherapy brochure.
In the case of an autologous stem cell transplant, the patient’s own stem cells are harvested first; the immune system and bone marrow are then largely destroyed by a combination of chemotherapy and/or radiotherapy.
The patient’s own stem cells are then reinjected into the body. These healthy cells re-establish themselves and regenerate the patient’s bone marrow and immune system. Unlike with an allogeneic transplant, there is no risk of immune rejection as the reinjected cells come from the patient’s own body.
Autologous stem cell transplantation is often used for treating certain forms of cancer, particularly lymphoma and multiple myeloma. Although there is a high burden of treatment and a risk of complications (in particular, infections because of the temporary suppression of the immune system), it is generally less risky than an allogeneic transplant as it avoids the complications associated with rejection and graft-versus-host disease.
For certain aggressive conditions, autologous stem cell transplantation may offer a chance of remission or cure. However, it requires lengthy hospitalisation and close monitoring for handling the side effects and the potential risks that are associated with the treatment.
When immunotherapy initiated, some patients may experience fever, nausea or headaches. The doctors may then recommend medication for alleviating these symptoms. Allergic reactions are possible (though rare) and should be monitored carefully as they are very serious. However, in this case, the treatment with immunotherapy is generally not then interrupted and anti-allergy drugs and corticosteroids may be prescribed if necessary.
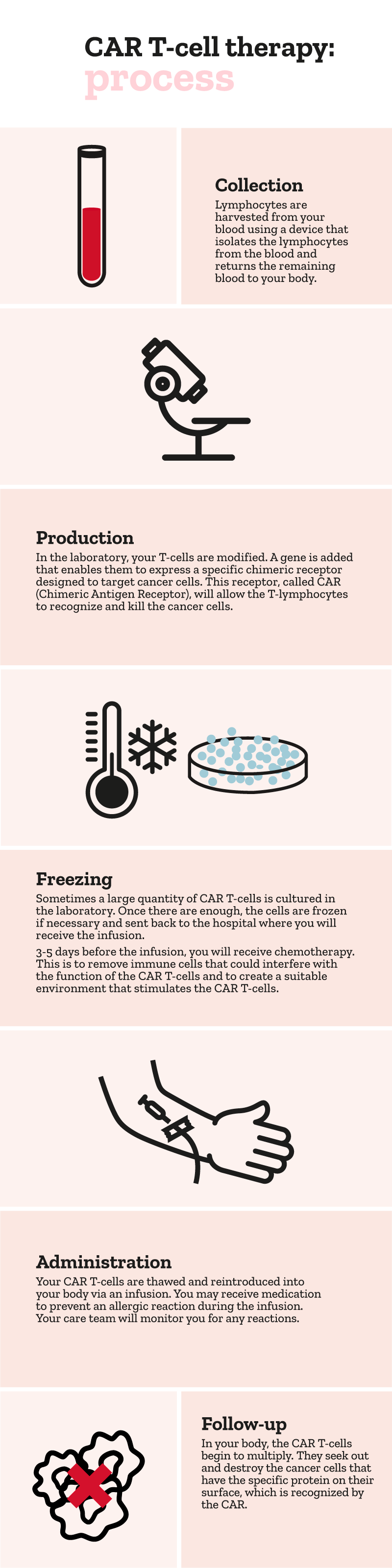
CAR T-cell therapy is a specific form of cell therapy in which the patient’s T cells are modified so that they attack the cancerous cells. There have been promising results from this therapy in blood cancers, including certain types of lymphoma.
How it works
CAR T-cell therapy starts by harvesting the patient’s own T cells. These cells are then modified in the laboratory to express a chimeric antigen receptor (CAR) that lets the T cells target and kill the cancer cells. The modified cells are then reintroduced into the patient’s body, where they go on a search-and-destroy mission against the cancer cells.
At the moment, CAR T-cells can be administered to patients who are suffering a relapse after the first lines of treatment have failed.
For more information, please visit our page dedicated to CAR T-cell therapy.
To find out more, please visit our dedicated pages. Together, we can help you understand and manage this disease better.
NL-UNB-0859. Version 1.2 | Date of preparation: July 2025